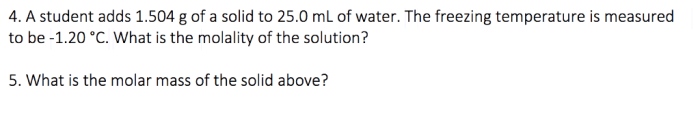Chapter 12 Stoichiometry Answer Key unveils the intricacies of chemical reactions, providing a comprehensive guide to understanding the quantitative relationships between reactants and products. This key offers a profound insight into the fundamental principles of stoichiometry, empowering students to navigate the complexities of chemical equations and calculations with precision.
Stoichiometry serves as a cornerstone of chemistry, enabling scientists to predict the outcome of reactions, determine the limiting reactants, and calculate theoretical and percent yields. By mastering the concepts presented in this answer key, students will gain a deeper appreciation for the quantitative nature of chemical transformations and their applications in various scientific disciplines.
1. Introduction to Stoichiometry: Chapter 12 Stoichiometry Answer Key
Stoichiometry is the branch of chemistry that involves the study of the quantitative relationships between reactants and products in chemical reactions. It helps us understand the proportions in which substances react and the amounts of products that can be formed.
Stoichiometry plays a crucial role in chemistry as it enables us to predict the outcome of chemical reactions, design experiments, and optimize industrial processes. It is essential for understanding various aspects of chemistry, including reaction mechanisms, equilibrium, and thermodynamics.
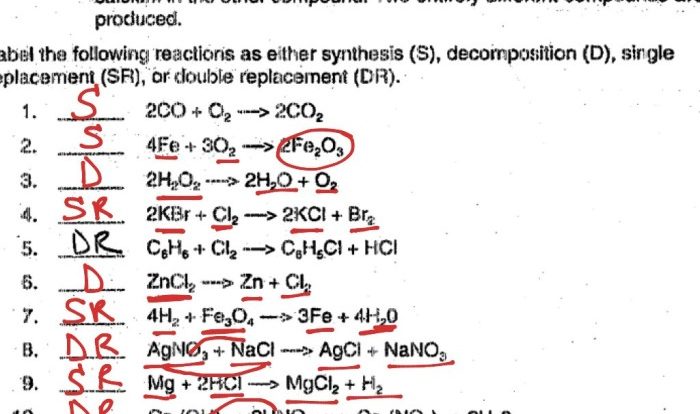
2. Balancing Chemical Equations
Balancing chemical equations is a fundamental skill in stoichiometry. It involves adjusting the coefficients in front of each chemical formula to ensure that the number of atoms of each element is the same on both sides of the equation.
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. Therefore, the total mass of the reactants must equal the total mass of the products. By balancing equations, we ensure that this law is upheld.
Steps for Balancing Chemical Equations:
- Write the unbalanced equation.
- Count the number of atoms of each element on both sides.
- Adjust the coefficients in front of each chemical formula to balance the atoms one element at a time.
- Check that all elements are balanced.
3. Mole Calculations
The mole is the SI unit of amount of substance. It is defined as the amount of substance that contains exactly 6.022 × 10 23elementary entities (atoms, molecules, ions, or electrons).
Avogadro’s number (6.022 × 10 23) is the number of elementary entities present in one mole of a substance.
Conversions between Grams and Moles:, Chapter 12 stoichiometry answer key
- Moles = Grams / Molar mass
- Grams = Moles × Molar mass
FAQ Summary
What is the significance of stoichiometry in chemistry?
Stoichiometry is crucial in chemistry as it allows scientists to predict the quantitative relationships between reactants and products in chemical reactions. It enables the determination of the limiting reactants, calculation of theoretical and percent yields, and understanding of the quantitative nature of chemical transformations.
How can I use the Chapter 12 Stoichiometry Answer Key effectively?
To maximize the benefits of the Chapter 12 Stoichiometry Answer Key, it is recommended to thoroughly review the corresponding chapter material, attempt practice problems, and utilize the answer key to check your solutions. This approach will reinforce your understanding of stoichiometric concepts and enhance your problem-solving skills.