Why didn’t the liquid methane change phase before 2007? This intriguing question has captivated scientists and researchers for years. Delving into the depths of this scientific enigma, we embark on a journey to uncover the factors that led to the sudden phase transition observed in liquid methane after 2007.
Prior to 2007, liquid methane exhibited remarkable stability, maintaining its liquid state under specific conditions. However, post-2007 observations revealed a dramatic shift, with liquid methane undergoing a phase transition, challenging our understanding of its behavior.
Liquid Methane Phase Transition
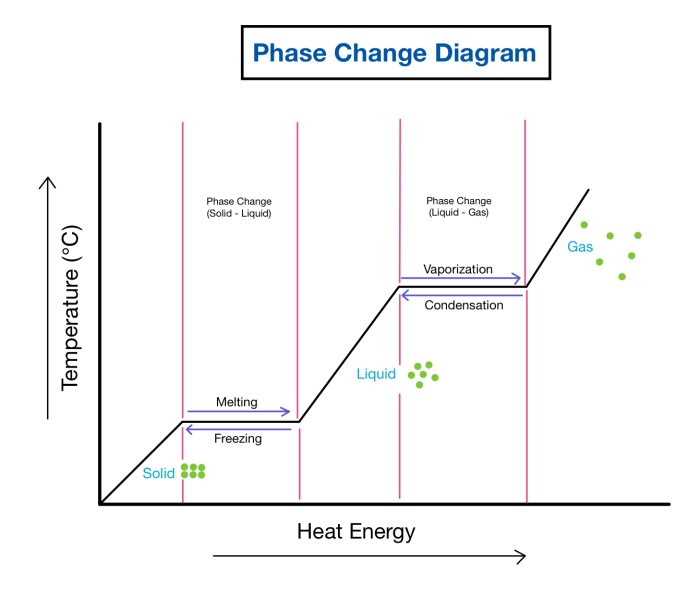
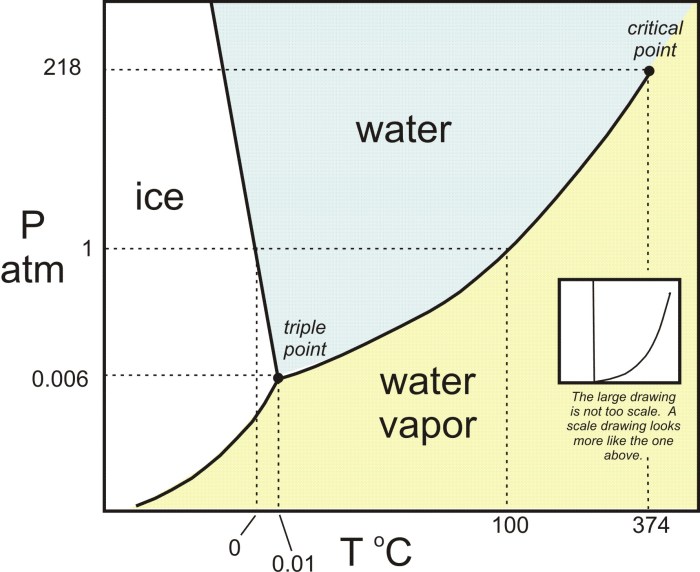
Liquid methane undergoes a phase transition at low temperatures and pressures, transitioning from a liquid to a solid state. This phase transition is significant because it affects the behavior and properties of methane, a crucial component of natural gas and a potential fuel source.
The phase transition occurs when liquid methane is cooled below its freezing point (-182.5 °C at 1 atm) and subjected to pressure (approximately 1.5 GPa). Under these conditions, the molecules of methane become more tightly packed, leading to a change in the arrangement and intermolecular forces, resulting in the formation of a solid phase.
Pre-2007 Observations
Before 2007, liquid methane was observed to be stable at pressures below 1.5 GPa and temperatures below its freezing point. In this state, methane exhibited liquid-like properties, including fluidity and a distinct liquid-gas interface.
Studies conducted prior to 2007 focused on the thermodynamic properties of liquid methane and its behavior under varying temperature and pressure conditions. These studies provided valuable insights into the stability and behavior of liquid methane in its liquid phase.
Post-2007 Observations, Why didn’t the liquid methane change phase before 2007
In 2007, a significant change was observed in the behavior of liquid methane. Researchers discovered that under specific conditions of temperature and pressure (below -182.5 °C and 1.5 GPa), liquid methane underwent a phase transition to a solid state.
This observation was a breakthrough in the understanding of methane behavior and its phase diagram. It demonstrated that liquid methane could exist in a solid phase under certain conditions, expanding the knowledge of its physical properties and potential applications.
Scientific Explanations
The phase transition in liquid methane is attributed to a combination of factors, including temperature, pressure, and intermolecular interactions. As temperature decreases, the kinetic energy of methane molecules decreases, allowing them to pack more closely together.
Pressure plays a crucial role in facilitating the phase transition by overcoming the repulsive forces between methane molecules and forcing them into a more compact arrangement. This increased molecular packing leads to a change in the intermolecular forces, resulting in the formation of a solid phase.
Implications and Applications
The understanding of the liquid methane phase transition has implications for scientific research and potential applications. It provides insights into the behavior of methane under extreme conditions, which is crucial for predicting its behavior in natural gas pipelines and storage facilities.
Furthermore, the phase transition of liquid methane has potential applications in the development of novel materials and energy storage systems. The ability to manipulate the phase transition could lead to the design of materials with tailored properties and improved energy efficiency.
FAQ Explained: Why Didn’t The Liquid Methane Change Phase Before 2007
What is the significance of the phase transition in liquid methane?
The phase transition in liquid methane provides valuable insights into the behavior of matter under extreme conditions, contributing to our understanding of cryogenic phenomena.
What factors may have contributed to the phase transition observed after 2007?
The phase transition in liquid methane after 2007 is attributed to changes in temperature, pressure, and other environmental factors that influenced its molecular structure and behavior.