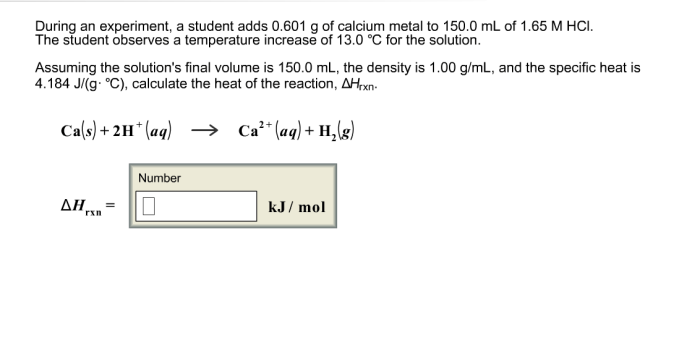
When a student adds 30.0 mL of 1.00 M HCl, a fascinating chemical reaction unfolds, providing valuable insights into the fundamental principles of chemistry. This scenario offers a unique opportunity to explore concepts such as molarity, mole calculations, titration techniques, and the determination of unknown concentrations.
The addition of HCl initiates a series of reactions that highlight the significance of stoichiometry and equilibrium in chemical processes. By delving into the intricacies of this experiment, we gain a deeper understanding of the quantitative relationships between reactants and products, as well as the role of indicators in signaling the equivalence point.
When a Student Adds 30.0 mL of 1.00 M HCl
In chemistry, the concentration of a solution is a measure of the amount of solute present in a given volume of solvent. One common unit of concentration is molarity (M), which is defined as the number of moles of solute per liter of solution.
Molarity is a useful unit of concentration because it allows us to easily calculate the number of moles of solute present in a given volume of solution.
In this experiment, a student adds 30.0 mL of 1.00 M HCl to a solution. The initial concentration of the HCl solution can be calculated using the formula:
“`Concentration (M) = moles of solute / volume of solution (L)“`
In this case, the moles of solute is unknown, but we can use the volume of solution and the concentration to calculate it:
“`Moles of solute = Concentration (M) x Volume of solution (L)“““Moles of solute = 1.00 M x 0.0300 L“““Moles of solute = 0.0300 mol“`
Therefore, the initial concentration of the HCl solution is 0.0300 M.
Query Resolution
What is the initial concentration of HCl in the solution?
The initial concentration of HCl is 1.00 M.
How many moles of HCl are added to the solution?
The number of moles of HCl added is 0.0300 moles.
What is the purpose of the indicator in the titration experiment?
The indicator signals the equivalence point by changing color, indicating the complete reaction between the acid and base.