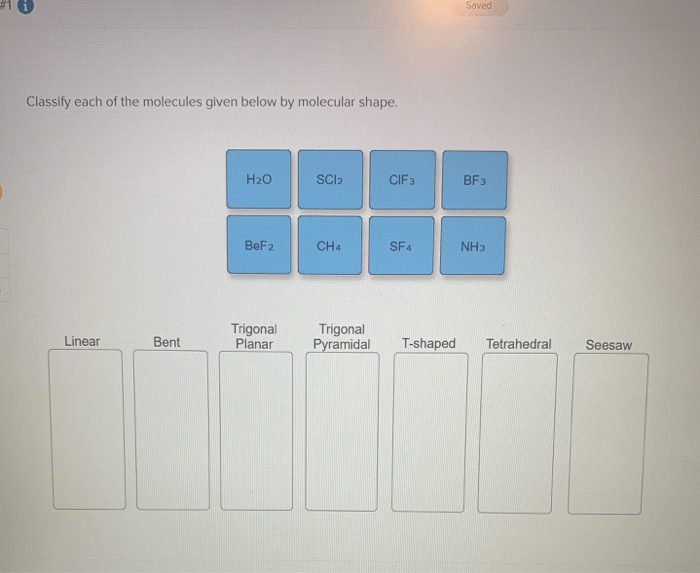
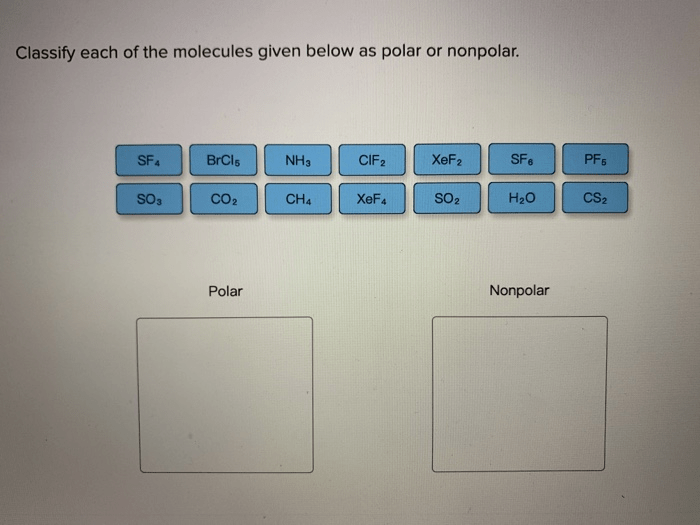
Classify each of the molecules given below by molecular shape, delving into the captivating world of molecular geometry. Molecular shape, a crucial aspect of molecular structure, holds the key to understanding the physical and chemical properties that govern the behavior of molecules.
By embarking on this journey of classification, we unlock the secrets of intermolecular interactions, reactivity, and selectivity, revealing the profound influence of molecular shape on the macroscopic world.
The determination of molecular shape hinges upon the VSEPR theory, a powerful tool that unveils the intricate dance of electron pairs and their profound impact on molecular geometry. As we delve deeper, we will explore the diverse array of molecular shapes, from the linear elegance of carbon dioxide to the tetrahedral symmetry of methane.
Each shape, a testament to the delicate balance of repulsive and attractive forces, holds a unique story, waiting to be unraveled.
Molecular Shape Classification: Classify Each Of The Molecules Given Below By Molecular Shape
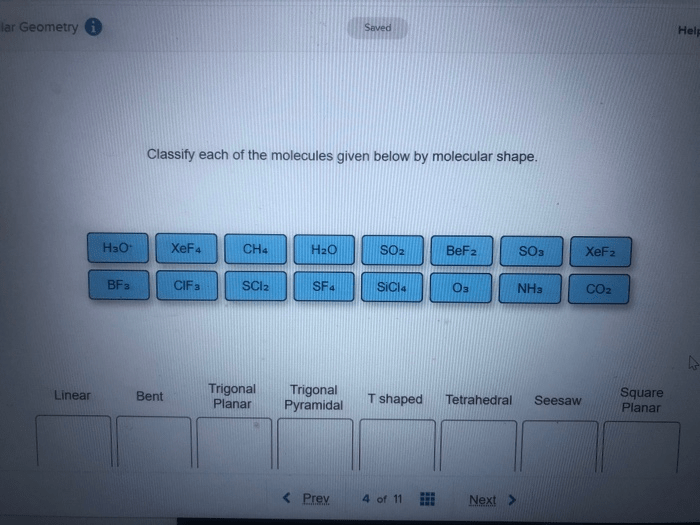
Molecular shape refers to the three-dimensional arrangement of atoms within a molecule. It is a crucial aspect of molecular structure that significantly influences physical and chemical properties. Classifying molecules by molecular shape provides valuable insights into their behavior and reactivity.
The VSEPR (Valence Shell Electron Pair Repulsion) theory is widely used to determine molecular shape. It states that electron pairs around a central atom repel each other, resulting in a molecular geometry that minimizes repulsion. The number and arrangement of electron pairs dictate the shape of the molecule.
Electron Pairs and Molecular Shape
Electron pairs can be bonding pairs (shared between atoms) or lone pairs (not shared). The number of bonding pairs and lone pairs around the central atom determines the electron pair arrangement, which in turn determines the molecular shape.
- Linear:2 bonding pairs, no lone pairs
- Trigonal Planar:3 bonding pairs, no lone pairs
- Tetrahedral:4 bonding pairs, no lone pairs
- Trigonal Pyramidal:3 bonding pairs, 1 lone pair
- Bent:2 bonding pairs, 1 lone pair
- Octahedral:6 bonding pairs, no lone pairs
- Square Pyramidal:5 bonding pairs, no lone pairs
- T-Shaped:5 bonding pairs, 1 lone pair
Classification of Molecules by Molecular Shape
| Molecule | Electron Pair Arrangement | Molecular Shape | Examples |
|---|---|---|---|
| CO2 | Linear | Linear | Carbon dioxide |
| NH3 | Trigonal Pyramidal | Trigonal Pyramidal | Ammonia |
| CH4 | Tetrahedral | Tetrahedral | Methane |
| H2O | Bent | Bent | Water |
| SF6 | Octahedral | Octahedral | Sulfur hexafluoride |
Applications of Molecular Shape Classification, Classify each of the molecules given below by molecular shape
Molecular shape classification is crucial for predicting various physical and chemical properties of molecules.
- Intermolecular Forces:Molecular shape influences intermolecular forces, such as dipole-dipole interactions and hydrogen bonding, which affect properties like melting point, boiling point, and solubility.
- Molecular Reactivity:Molecular shape can affect molecular reactivity and selectivity. For instance, linear molecules tend to be more reactive than branched molecules in certain reactions.
Clarifying Questions
What is the significance of molecular shape?
Molecular shape dictates intermolecular interactions, influencing properties such as solubility, boiling point, and reactivity.
How does VSEPR theory determine molecular shape?
VSEPR theory predicts molecular shape by considering the repulsive forces between electron pairs, minimizing their overall energy.
What are the common molecular shapes?
Common molecular shapes include linear, trigonal planar, tetrahedral, and octahedral, each resulting from specific electron pair arrangements.