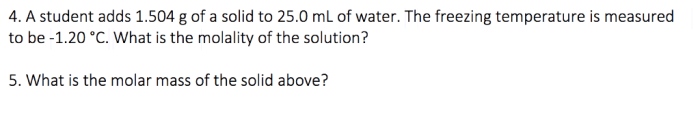Introducing the gizmo reaction energy answer key, your ultimate guide to understanding the fascinating world of chemical reactions and the energy that drives them. This comprehensive resource will take you on a captivating journey, unlocking the secrets of reaction energy and its significance in various fields.
As we delve into the depths of this topic, we’ll explore the concept of reaction energy, its formula, and the factors that influence it. We’ll also investigate the practical applications of reaction energy, showcasing its importance in energy production, industrial processes, and beyond.
Gizmo Reaction Energy Overview
Reaction energy refers to the energy change associated with chemical reactions. It is the difference between the energy of the reactants (initial substances) and the energy of the products (final substances). Reaction energy is crucial in determining the spontaneity and feasibility of chemical reactions.
Gizmo Simulation
The Gizmo simulation is an interactive tool designed to explore reaction energy. It allows users to simulate various chemical reactions and observe the corresponding energy changes. The simulation provides a visual representation of the energy profile of reactions, helping users understand the concepts of activation energy, exothermic reactions, and endothermic reactions.
Gizmo Reaction Energy Experiment
In this experiment, you will use the Gizmo to investigate the energy changes that occur during chemical reactions. You will manipulate variables such as the type of reactants, the concentration of the reactants, and the temperature of the reaction. You will then collect data on the amount of heat that is released or absorbed during the reaction.
The Gizmo is a virtual laboratory that allows you to perform experiments safely and easily. The Gizmo includes a variety of tools that you can use to manipulate the variables of the experiment and collect data.
Procedure
- Open the Gizmo Reaction Energy.
- Select the “Reactants” tab.
- Choose two reactants from the drop-down menus.
- Click on the “React” button.
- Observe the reaction that takes place in the reaction chamber.
- Click on the “Data” tab.
- Record the values of the following data:
- Initial temperature
- Final temperature
- Heat released or absorbed
- Repeat steps 3-7 for different combinations of reactants.
- Analyze the data to determine the effect of the following variables on the amount of heat released or absorbed during the reaction:
- Type of reactants
- Concentration of the reactants
- Temperature of the reaction
Reaction Energy Calculations: Gizmo Reaction Energy Answer Key
Reaction energy (ΔH) is the change in enthalpy that occurs during a chemical reaction. It is the difference between the enthalpy of the reactants and the enthalpy of the products.
The formula for calculating reaction energy is: “` ΔH = H products– H reactants“` where:
- ΔH is the reaction energy
- H productsis the enthalpy of the products
- H reactantsis the enthalpy of the reactants
Factors Affecting Reaction Energy
Reaction energy, the energy change accompanying a chemical reaction, is influenced by various factors. These factors determine the favorability and outcome of reactions.
Bond Strength, Gizmo reaction energy answer key
The strength of bonds formed and broken during a reaction plays a crucial role. Reactions that result in the formation of stronger bonds tend to release energy (exothermic), while those breaking stronger bonds require energy input (endothermic). Bond strength is measured by bond energy, and reactions proceed spontaneously when the total bond energy of the products exceeds that of the reactants.
Temperature
Temperature affects reaction rates and equilibrium positions. Increasing temperature generally favors endothermic reactions by providing the necessary energy to break bonds. Conversely, decreasing temperature shifts the equilibrium towards exothermic reactions, promoting bond formation.
Concentration
The concentration of reactants affects reaction rates. Higher concentrations lead to increased collisions between reactant particles, increasing the likelihood of successful reactions. This effect is particularly significant in gas-phase reactions, where the number of collisions is directly proportional to concentration.
Applications of Reaction Energy
Reaction energy plays a crucial role in various fields, enabling technological advancements and industrial processes.
In energy production, reaction energy is harnessed to generate electricity. Fossil fuels, such as coal and natural gas, undergo combustion reactions, releasing energy that drives turbines connected to electricity generators. Nuclear reactions, such as those in nuclear power plants, also release immense amounts of energy that can be converted into electricity.
Industrial Processes
- Manufacturing:Reaction energy is utilized in the production of numerous materials, including steel, cement, and glass. These processes involve chemical reactions that require significant energy input, often achieved through the combustion of fuels or electrical heating.
- Food Processing:Reaction energy is essential in food processing to modify and preserve food products. Canning, freezing, and dehydration are examples of processes that utilize reaction energy to extend shelf life and enhance food quality.
- Pharmaceuticals:The synthesis of pharmaceuticals relies heavily on reaction energy. Chemical reactions are carefully controlled to produce specific molecules with desired therapeutic effects.
Troubleshooting and Tips
The Gizmo simulation is a powerful tool for exploring reaction energy, but it is important to be aware of some common errors and difficulties that can occur. Here are some troubleshooting tips to help you get the most accurate results from your experiments:
- Make sure that you are using the correct units.The Gizmo simulation uses kilojoules per mole (kJ/mol) for reaction energy. If you are using a different unit, you will need to convert your results before comparing them to the expected values.
- Be careful when measuring the reactants and products.Even small errors in measurement can lead to significant errors in your results. Use a graduated cylinder or balance to measure the reactants and products as accurately as possible.
- Allow the reaction to complete before measuring the products.If you measure the products too early, you may not get an accurate reading of the reaction energy.
- If you are having trouble getting a consistent reading, try repeating the experiment several times.The more data you have, the more accurate your results will be.
Key Questions Answered
What is reaction energy?
Reaction energy is the energy change that occurs during a chemical reaction. It can be either released (exothermic reaction) or absorbed (endothermic reaction).
How do I calculate reaction energy?
Reaction energy can be calculated using the formula ΔH = H(products) – H(reactants), where ΔH represents the change in enthalpy, H(products) is the total enthalpy of the products, and H(reactants) is the total enthalpy of the reactants.
What factors affect reaction energy?
Factors that affect reaction energy include bond strength, temperature, concentration, and the presence of a catalyst.